|
This is a file from the Wikimedia Commons. Information from its description page there is shown below.
Commons is a freely licensed media file repository. You can help.
|
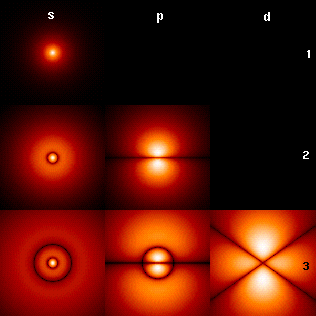
English: First few hydrogen atom orbitals; cross section showing colour-coded probability density for different n=1,2,3 and l="s","p","d"; note: m=0
The picture shows the first few hydrogen atom orbitals (energy eigenfunctions). These are cross-sections of the probability density that are colour-coded (black=zero density, white=highest density). The angular momentum quantum number l is denoted in each column, using the usual spectroscopic letter code ("s" means l=0; "p": l=1; "d": l=2). The main quantum number n (=1,2,3,...) is marked to the right of each row. For all pictures the magnetic quantum number m has been set to 0, and the cross-sectional plane is the x-z plane (z is the vertical axis). The probability density in three-dimensional space is obtained by rotating the one shown here around the z-axis.
Note the striking similarity of this picture to the diagrams of the normal modes of displacement of a soap film membrane oscillating on a disk bound by a wire frame. See, e.g.,
Vibrations and Waves, A.P. French, M.I.T. Introductory Physics Series, 1971, ISBN 0393099369, page 186, Fig. 6-13. See also Normal vibration modes of a circular membrane.
Slovenščina: Valovne funkcije elektrona v vodikovem atomu imajo določeno energijo (naraščajoče od zgoraj: n=1,2,3 ...) in vrtilno količino (naraščajoče prek: s, p, d ...). Svetlejša področja odgovarjajo višji verjetnostni gostoti za merjenje lege. Vrtilna količina in energija sta kvantizirani in zavzemata le nezvezdne vrednosti, kot jih kažejo slike.
Originally uploaded to :en by en:User:FlorianMarquardt at 18:33, 14 Oct 2002.
 |
Permission is granted to copy, distribute and/or modify this document under the terms of the GNU Free Documentation License, Version 1.2 or any later version published by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts, and no Back-Cover Texts.
Subject to disclaimers.www.gnu.org/copyleft/fdl.htmlGFDLGNU Free Documentation Licensetruetrue
|
File usage
The following pages on Schools Wikipedia link to this image (list may be incomplete):
Wikipedia for Schools is one of SOS Children's many educational projects. SOS Children believes that a decent childhood is essential to a happy, healthy. Our community work brings families new opportunities through education, healthcare and all manner of support. Have you thought about sponsoring a child?